Zinc blende has a giant crystal lattice structure with strong electrostatic forces of attraction between zinc and sulphur in an tetrahedral structure.
As such, a blast furnace is required to extract zinc from zinc blende.
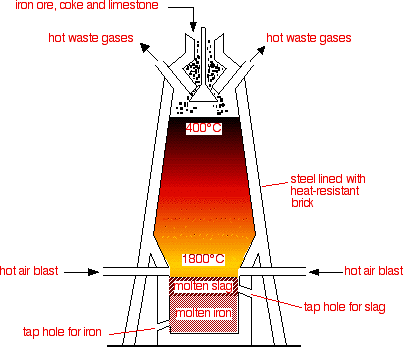
Using the Blast Furnace
In order to prevent carbon
monoxide from forming, an alternative method, electrolysis can be used when done will not release any harmful substances into
the environment.
Zinc sulphide is oxidized by oxygen to
produce zinc oxideand sulphur dioxide.
2ZnS(s) + 3O2(g) è ZnO(s) + 2SO2(g)
Most of the zinc in the zinc sulphide are
oxidized form Zinc Oxide. Some of the zinc reacts with iron to form zinc
ferrite.
Sulphur dioxide is then further processed to
produce sulphuric acid.
Zinc oxide is then mixed with concentrated
sulphuric acid to produce zinc sulphate.
ZnO(s) + H2SO4 (aq) è ZnSO4 (aq) + H2O(l)
The end product will be water and water will not be
harmful to the environment.
Links:
http://www.chemguide.co.uk/inorganic/extraction/iron.html
http://chem-guide.blogspot.sg/2010/04/occurrence-and-extraction-of-zinc-from.html
http://kennychemistry.blogspot.sg/2012/09/extraction-of-zinc-from-zinc-blende.html
http://www.cpsc.gov/cpscpub/pubs/466.html
http://en.wikipedia.org/wiki/Sphalerite
This comment has been removed by the author.
ReplyDelete